Details of the Drug
General Information of Drug (ID: DMM4QOJ)
| Drug Name |
Metadoxine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | MG01CI; Metadoxine (oral sustained release, ADHD); Metadoxine (oral extended release, ADHD), Alcobra; Metadoxine (oral sustained release, ADHD), Alcobra | ||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
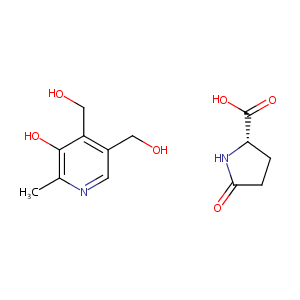 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 298.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 02 Neoplasm | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: 2C82 Prostate cancer | |||||||||||||||||||||||
| The Studied Tissue | Bone marrow | |||||||||||||||||||||||
| The Studied Disease | Multiple myeloma [ICD-11:2C82] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
